Glyciome Awarded Landmark Patent for Prebiotic Cervical Cancer Prevention Approach Focused on Under-Served Populations
Novel lubricant aims to optimize vaginal ecosystem health and limit product-use that increases BV and STI risks associating with cancer.
“Cervical cancer has a clear socioeconomic bias, with mortality rates rising, especially among US women in low-income counties” said Glyciome CEO and product co-inventor, Dr. Joanna Ellington. “Those most impacted populations include rural white women, Mexican Americans, non-Hispanic Black Americans, Puerto Ricans, American Indians, and women service members. Reproductive care is often stigmatized in these communities, creating barriers to treatment.” Glyciome’s groundbreaking patent covers both the prebiotic lubricant and a discreet delivery system, designed to optimize adoption even for those without access to clean water or waste management.
The patented lubricant delivery kit includes the first-in-kind vaginal lubricant, innovative kraft-paper applicators, and a discreet “all-in, all-out” dispensing and waste-collection carton designed for incineration, ensuring maximum user privacy. Glyciome is committed to developing products to reduce infections linked to genital cancers. The kraft-paper applicator not only meets rigorous standards for effective gel delivery but also offers a sustainable alternative, reducing women's exposure to harmful plastic chemicals associated with cancer risks. Additionally, the prebiotic lubricant is the mildest of lubricants tested to date by third parties in preclinical studies, showing a lack of harm to vaginal cells or healthy vaginal bacteria.
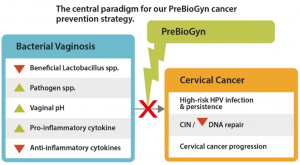
Unlike current BV approaches that disrupt vaginal function and can increase risks of STI and BV recurrence, Glyciome’s approach supports the healthy vaginal ecosystem. Although high-risk human papillomavirus (HPV) vaccines can decrease cancer rates, only ~55% of US teens and fewer than 20% of older women are fully vaccinated. HPV alone doesn’t cause cervical cancer; it is the inflammation of BV together with the HPV virus that triggers formation and progression of this cancer. Cervical cancer is the fourth most common cancer among women globally, with maternal deaths leading to as many as 10% of global orphans. In the US, with 300,000 people living with this cancer and death rates rising, novel prevention methods are urgently needed.
“About 34 million Americans have BV. Current treatments, including oral antibiotics, result in a 40% recurrence rate, while over-the-counter products, such as symptom-management lubricants, are sold without FDA review of marketed claims around vaginal ecosystem health” said co-inventor and Doctor of Pharmacy, GD Clifton. “Two decades of published studies warn that lubricants, and even natural oils, can increase STI and BV risks by harming beneficial vaginal bacteria and increasing bad bacterial types that result in unwanted ‘fishy’ odors and inflammation associating with cancer. This increased risk from lubricant use led to the UN convening a recent global consultation on safer product development, which our product was designed to address.”
Glyciome’s team, spanning Spokane WA, Boston MA, and Puerto Rico, has spent more than four years developing their novel lubricant for at-home and local healthcare use, focusing on diverse populations. “Approximately 25% of American women are looking for an alternative to HPV vaccines for cervical cancer prevention,” said Dr. Jenny Fremlin, Glyciome’s digital director and diversity ambassador. “Our product fills that need, especially for women whose personal healthy vaginal bacteria don’t match the strains provided in most probiotics, and for those who aren’t getting sex education in schools or feel like what they’re being taught doesn’t really relate to them.”
Glyciome founders, who previously invented PreSeed®, the first FDA-approved sperm-safe lubricant for trying-to-conceive couples, have now turned their attention to cervical cancer prevention. Dr. Ellington’s PhD studies at Cornell included International Development, providing insight into challenges for LRS reproductive healthcare. She explains, “The UN identifies pregnant women as those most at risk for cervical cancer, and they are a group we hope our product will especially benefit. We are also very proud to have received product development funding through the National Cancer Institute in a small-business Seed grant, and a regional Health Sciences & Services Authority of Spokane County grant, which has helped us advance our technology toward this trailblazing achievement.” (R41CA254543).
Joanna Ellington
Glyciome LLC
+1 509-828-0655
info@glyciome.com
Visit us on social media:
Facebook
Instagram
YouTube
TikTok
Distribution channels: Beauty & Hair Care, Consumer Goods, Culture, Society & Lifestyle, Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release