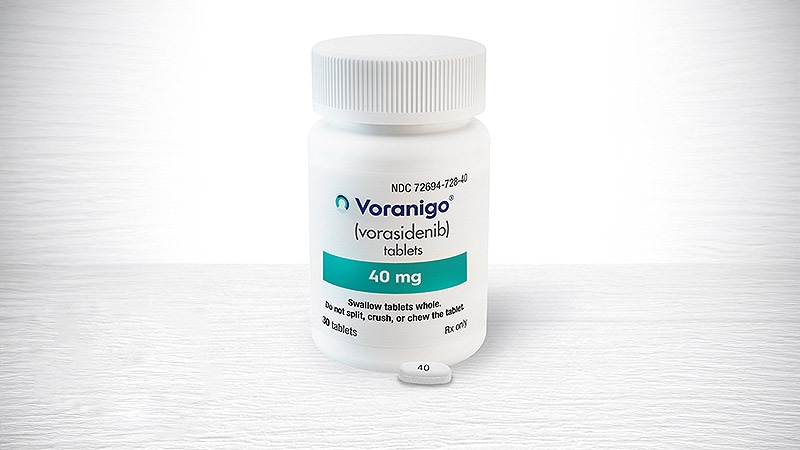
EMA Greenlights Voranigo for IDH-Mutant Grade 2 Glioma
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended granting marketing authorization in the European Union to Voranigo (vorasidenib; Les Laboratoires Servier), for treatment of predominantly …