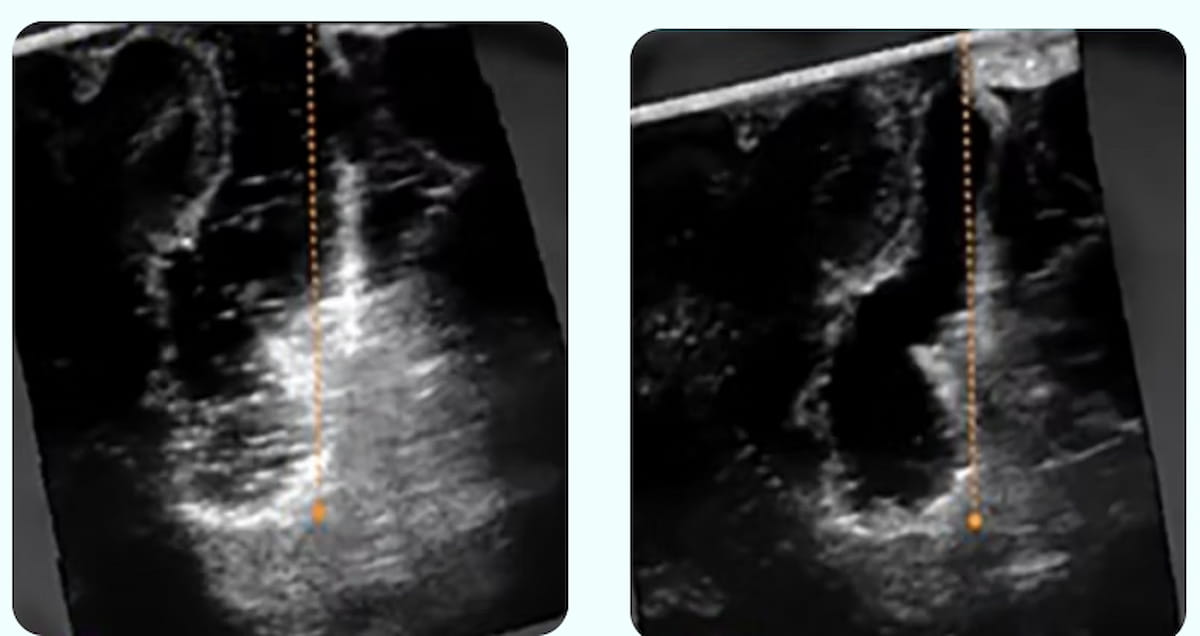
SonoClear System Gets FDA Breakthrough Device Designation for Enhancing Ultrasound-Guided Brain Tumor Resection
The Food and Drug Administration (FDA) has issued a breakthrough device designation for the SonoClear System, an acoustic coupling fluid and sterile transfer kit that improves the clarity of intraoperative ultrasound in brain tumor surgery. In contrast to …