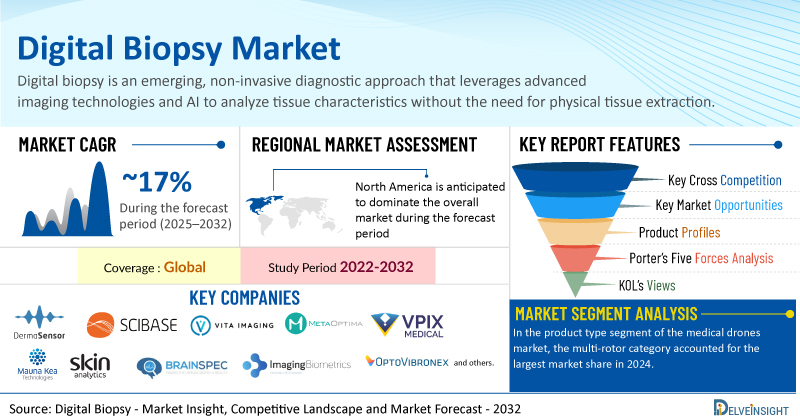
Europe's CHMP gives thumbs-up to Lilly's Kisunla and Gilead's long-acting PrEP drug Yeytuo
Four months after giving Eli Lilly’s Alzheimer’s disease treatment a thumbs-down, Europe’s Committee for Medicinal Products for Human Use (CHMP) has recommended it for approval, albeit in a restricted patient population. The positive opinion covers Kisunla …